Just The Facts: Contaminants are Everywhere
Indoor cannabis flowering environments are biologically dense ecosystems, hosting extensive populations of yeasts and molds. Flowering plants, room air, and structural surfaces together support very large microbial populations despite visually clean conditions and well-managed cultivation practices. It is common knowledge that regulatory microbial testing measures colony-forming units (CFU) per gram of dried flower. What is not readily apparent is that this represents only a fraction of the viable fungal biomass in a cultivation environment. This blog outlines the conceptual and quantitative framework for estimating total yeast and mold load in a typical indoor cannabis flower room, linking CFU per gram to per-plant totals in addition to scaling across both canopy concentration and room-level air.
The Flowering Cycle: Growing Much More Than Cannabis
During the last week of flowering, contamination risk multiplies as living cannabis flowers carry substantial surface and internal microbial communities. Outer flower tissues, including bracts and calyces, are heavily colonized, while leaves and stems harbor lower populations. Microbial populations accumulate and increase during the flowering cycle due to a few factors including natural exudation, canopy humidity, and low UV exposure in indoor environments. The living flower provides multiple microhabitats for yeast and mold:
- outer surfaces colonized by spores and vegetative cells
- trichome bases rich in sugars supporting microbial metabolism
- calyx interiors sheltering endophytic molds
- stigmas and pistils that may serve as entry points for fungal invasion.
This combination of surface and internal colonization establishes the flower as a biological surface that supports both epiphytic and endophytic organisms.
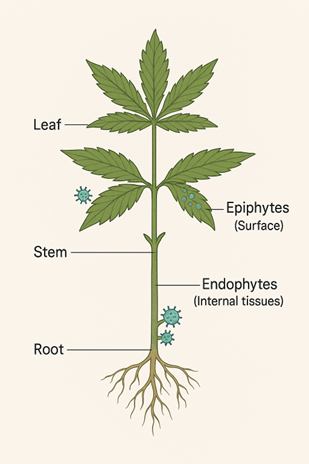
How much yeast and mold are found on cannabis flowers vs the whole plant?
When labs test cannabis flowers, they are measuring dry material which reduces the CFU count by a factor of 10 to 100 compared to preharvest material. During late flowering, total yeast and mold populations on living flower tissue typically range from 100,000 to 1,000,000 CFU per gram, with higher densities observed in humid or poorly ventilated rooms. This difference is only part of the story.
When you know how much flower a plant produces, you can estimate the total number of microbes on the whole plant. For example, if a plant produces 300 grams of flower, and each gram is hosting 500,000 CFU, then the plant carries roughly 150 million CFU on the flowers alone. Add the leaves, stems, and other parts of the plant, and the total number of CFU can sometimes climb beyond a billion per plant.
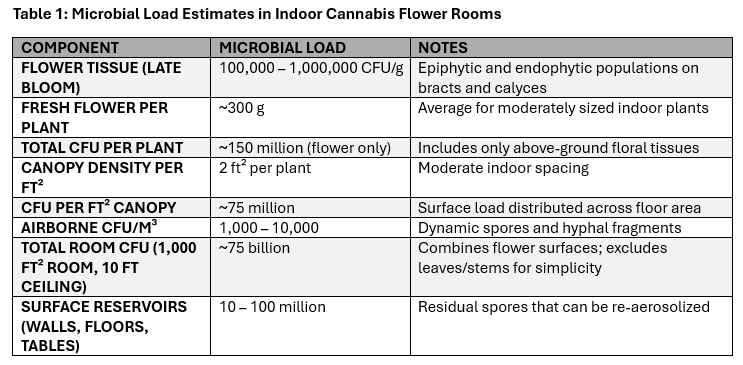
How does plant spacing affect mold and yeast levels?
The way plants are spaced in a grow room affects how many microbes (like mold and yeast) are present in each square foot of space. In tightly packed setups, like a “sea of green” where each plant only takes up about one square foot, increased plant density creates increased CFU counts.
To estimate the CFU in a defined space, multiply the number of microbes per plant by the number of plants in that space. A typical indoor grow room with moderate plant density can yield tens of millions of microbes in each square foot of canopy. As an example, the average 1,000 square ft grow room can host tens of billions of mold and yeast cells on plant surfaces alone.
Microbes live on the surface and within plants, as well as in the air. Airborne mold, in the form of spores and hyphal fragments, travels throughout grow rooms, floating through the canopy with ease. In a controlled indoor grow room, the air typically contains hundreds to thousands of live mold and yeast cells per cubic meter. These come from the plants themselves, from pruning and maintenance, and from air systems that recirculate spores.
Even though there are fewer microbes in the air than there are on the plants, the air is the primary source of distribution and therefore, contamination. If the humidity is high or the air is not moving well, the number of airborne spores increases, which can lead to greater concentrations of microbial contamination overall.

How does the environment affect mold and yeast in grow rooms?
The conditions inside a grow room—like humidity, temperature, and airflow—have a significant impact on how much mold and yeast are present. When the humidity rises above 60%, mold spores can start to germinate in as little as 12 hours. Closely packed plants create damp micro-environments where mold can thrive, while warmer temperatures speed up the metabolic rate of microbes; these conditions promote faster multiplication.
Air circulation has a complex effect. While it reduces humidity, it also disperses spores. This highlights the need for high-efficiency filtration along with regular cleaning and disinfection to reduce both airborne and surface populations.
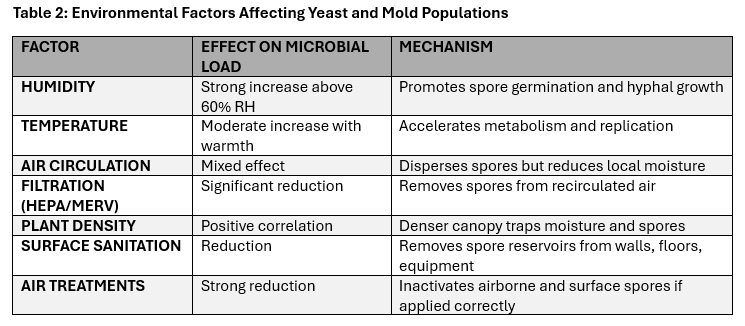
Summary
Even under visually clean conditions and well-managed cultivation practices, flowering plants, room air, and structural surfaces together support very large microbial populations. While most of the mold and yeast microbes live on and in the plants, it is essential to focus on the environment to combat their overall proliferation. Contaminants travel through the air. Spores build up on walls, floors, and equipment. Activities such as pruning or cleaning can redistribute spores throughout a contained space. Even in a well-maintained grow room, all these sources combined can add up to more than 100 billion CFU in a space as small as 1,000 square feet.
This raises a key point: TYM and PCR testing capture only part of the microbial potential. These tests focus on the dried flower, missing the environmental and growing conditions surrounding it. The key to managing mold and yeast is a holistic approach:
- ensure humidity below 60%
- maintain filtered and clean air circulation
- clean and disinfect regularly
Understanding the distribution of CFU across flower tissue, plant canopy, air, and surfaces allows growers and regulators to conceptualize microbial “pressure” in the cultivation environment and identify the most effective interventions for compliance and product safety.
References
- Punja, Z. K. (2023). Total yeast and mold levels in high THC-containing cannabis inflorescences are influenced by genotype, environment and pre- and post-harvest handling practices. PMC. PMC
- Punja, Z. K. (2023). Organically grown cannabis (Cannabis sativa L.) plants… Canadian Journal of Botany (or similar) Canadian Science Publishing
- Prussin, A. J. II & Marr, L. C. (2015). Sources of airborne microorganisms in the built environment. Microbiome. BioMed Central
- Gwinn, K. D. et al. (2023). Fungal and mycotoxin contaminants in cannabis and hemp. PMC
- Buirs, L. (2025). Endophytes in Cannabis sativa: Identifying and Characterizing Microbes with Beneficial and Detrimental Effects. MDPI Plants. MDPI


